Introduction: Autologous hematopoietic stem cell transplantation (auto-HCT) is the standard treatment for patients with chemo-sensitive relapsed/refractory diffuse large B cell lymphoma (DLBCL). However, post-auto-HCT outcomes are still poor in this population, with 5-year progression free survival (PFS) of 40%. We hypothesize that in patients with DLBCL, blinatumomab consolidation post auto-HCT will eradicate remaining tumor cells, leading to decreased relapse and increased overall survival. Therefore we conducted a pilot study to test blinatumomab as consolidation therapy post auto-HCT for patients with DLBCL.
Methods: Adult patients with chemosensitive DLBCL or transformed FL who underwent auto-HCT were included. All patients received one cycle of blinatumomab consolidation starting 42 days post auto-HCT (9 mcg daily as continuous infusion for 7 days, followed by 28 mcg daily for 21 days). Response evaluation was done at day 100 post auto-HCT. Minimal residual disease (MRD) was quantified by immunoglobulin high-throughput sequencing (Ig-HTS) of plasma cell-free DNA on days 42 post auto-HCT (pre-blinatumomab) and on day 100 post auto-HSCT (one month post completion of blinatumomab). Immunophenotyping of T cells in cryopreserved peripheral blood mononuclear cells collected on day 42 (pre-blinatumomab), day 56 (midpoint of blinatumomab treatment cycle), and day 100 (1 month post blinatumomab) was performed using 18-color flow cytometry panels for extracellular and intracellular antigens.
Results: As of August 2020, ten patients have been treated with at least 100 days follow up. Patient characteristics and outcomes are summarized in Table 1. Three out of 10 patients (30%) were in partial remission (PR) as determined by CT or PET/CT imaging before auto-HCT. All subjects completed the planned cycle of blinatumomab consolidation. Blinatumomab was well tolerated. Two patients developed grade 1 CRS, with no grade 2 and higher CRS. Immune effector cell-associated neurotoxicity syndrome (ICANS) was not observed. Six patients developed transient tremor (four grade 1, one grade 2, and one grade 3). One subjects developed BCNU pneumonitis and CMV viremia that resolved with steroid and ganciclovir.
One hundred days post auto-HCT (one month post blinatumomab consolidation) 10/10 (100%) of patient were in complete remission (CR) as determined by both MRD testing and by CT or PET/CT imaging. Plasma cell free based MRD was positive on day 42 (post auto-HCT and pre-blinatumomab), in two out of ten patients (20%). These two patients achieved MRD negative status after receiving blinatumomab consolidation. With median follow up of 14.5 months (range: 7-34 months), all 10 patients are alive and 6/10 remain in remission..
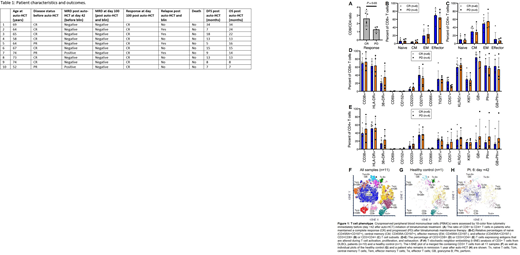
Interestingly, the 4 patients with disease relapse had lower CD8/CD4 T cell ratio before starting blinatumomab compared with patients who remained in remission (Figure 1A). However, there were no significant differences in the distribution of the major T cell subtypes (naïve, memory, effector and Treg), and expression of markers of T cell activation, proliferation, or exhaustion (Figure 1B-1E). High dimensional analysis with t-stochastic neighbor embedding (tSNE) revealed a cluster of CD8+ and CD4+ T cells characterized by high expression of granzyme B (GB) and perforin that was present in the DLBCL patients before and after blinatumomab treatment but not in a healthy untreated control (Figure 1F-1H). Although further analysis of healthy untreated controls and pre-transplant samples is needed, CD8+ T cells from these DLBCL patients pre-blinatumomab contained very few naïve cells and were enriched for terminally differentiated effector cells.
Conclusion: This pilot study shows that blinatumomab consolidation post auto-HCT is safe and well tolerated. MRD response to blinatumomab in all patients with MRD positive disease post auto-HCT is encouraging. Strategies to increase CD8/CD4 ratio and more cycles of consolidation in a larger randomized trial are needed to confirm the efficacy of consolidation with blinatumomab post auto-HCT. Finally, the unusually "high activation" immunophenotype (Teff/GB+) seen in CD8 T cells of DLBCL patients after auto-HCT (compared to those seen in resting peripheral blood) may both impact the response to blinatumomab and provide key insights into optimal timing for administration after auto-HCT.
Ghobadi:WuGen: Consultancy; Bristol Myers Squibb: Consultancy; Kite: Consultancy, Research Funding; EUSA: Consultancy; Amgen: Consultancy, Research Funding. Mehta-Shah:Bristol Myers-Squibb: Research Funding; Karyopharm Therapeutics: Consultancy; Corvus: Research Funding; Genetech/Roche: Research Funding; Innate Pharmaceuticals: Research Funding; Kyowa Hakko Kirin: Consultancy; Celgene: Research Funding; Verastem: Research Funding; C4 Therapeutics: Consultancy. Kahl:Celgene Corporation: Consultancy; AstraZeneca Pharmaceuticals LP: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC: Consultancy; Roche Laboratories Inc: Consultancy; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy; AbbVie: Consultancy. DiPersio:Magenta Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal